Experience as part of the Negotiation team for the Free Trade Agreement between Mexico and the EU in Agri-food and spirits access, Sanitary and Phytosanitary Measures (SPS) and Geographical indications. Regarding the SPS we participated on the pre-listing, audit visits, among other issues.
We have the experience on the field, from 2014 to 2019 on Planning, Organising and Preparing Inspection Visits from the EU to Mexico on Bovine and equine meat. As well as the inspection visits from Mexico to the EU on pork meat.
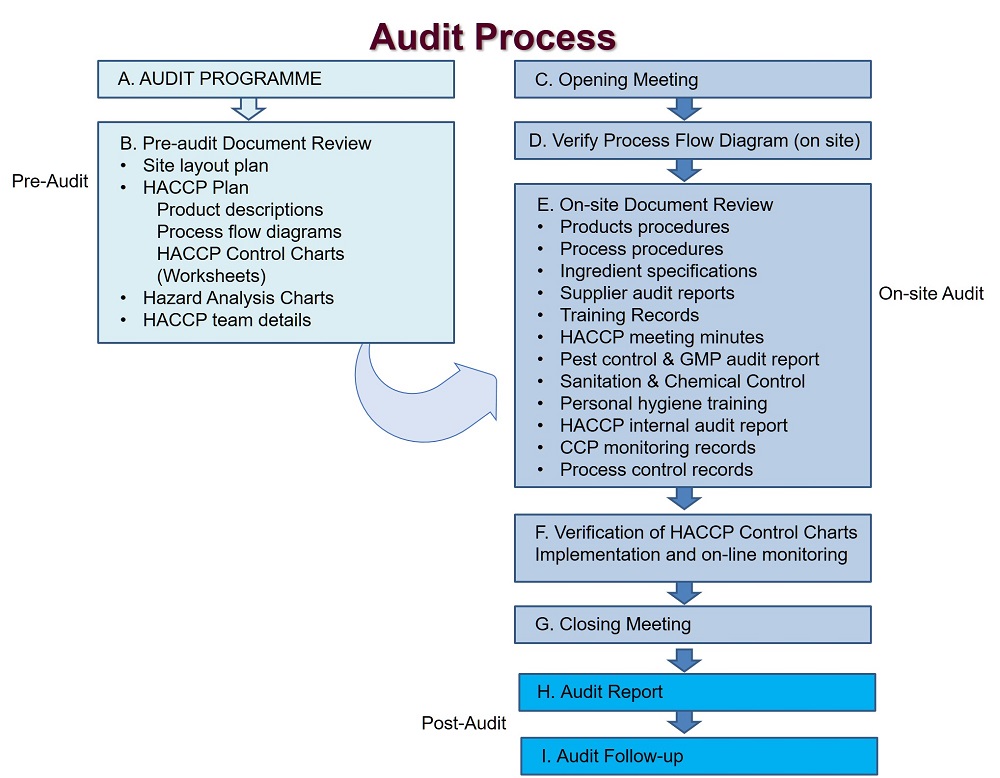
We could help you to implement a Food Safety Management System and also audit your actual System, we especialize on slaughterhouse, cutting and deboning complying with EU, USA and Mexico Regulations.
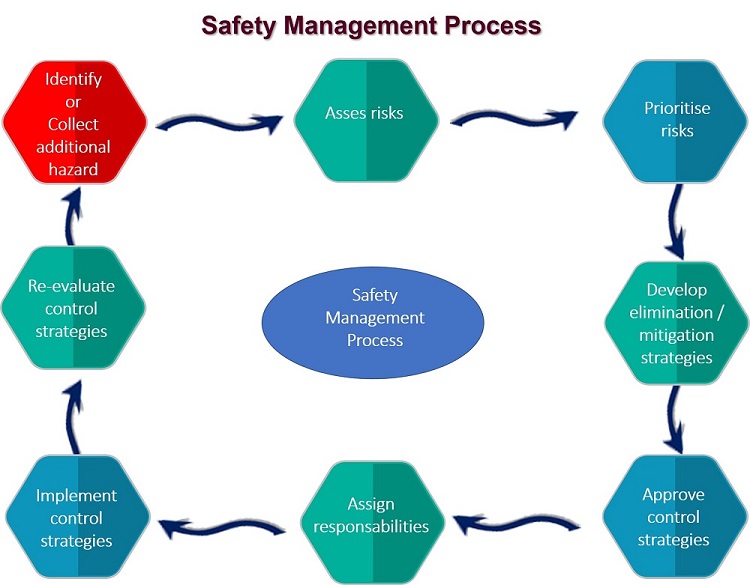
A food safety management system is a group of procedures and practices that work together to prevent foodborne illness.
It does this by actively controlling risks and hazards throughout the flow of food.
There is concern on Food Safety Hazards among consumers and also among producers.
Caused by:
Consumers and Producers want to prevent food hazards and incidents.
The Food and Drug Administration has identified five risk factors that contribute to most foodborne illnesses in the U.S.
Programs that should be in place:
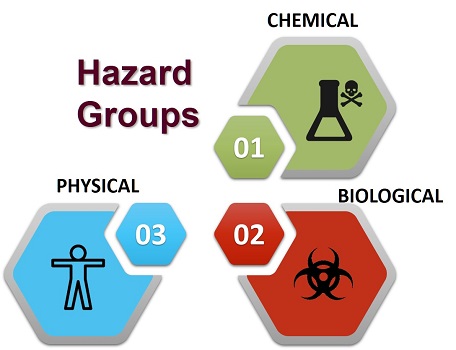
"A biological, chemical or physical agent in, or condition of, food with the potential to cause an adverse health effect"
(Codex Alimentarius Commission)
"A condition or physical situation with a potential for an undesirable consequence"
a situation involving exposure to danger
the possibility that something unpleasant will happen
is the chance, high or low, that any hazard will actually cause somebody harm.
Hazard: something with the potential to cause harm.
Risk: the likelihood of occurrence and the magnitude of consequences of a specified hazard being realized
There are many hazards associated with food that can and do result in injury and harm to human health.
Millions of people worldwide suffer from some sort of "food poisoning" each year.
Uncontrolled application of agricultural chemicals, environmental contamination, use of unauthorized additives, microbiological hazards and other abuses of food along the food chain can all contribute to the potential of introducing or failing to reduce hazards related to food.
With increased awareness of the effects of food hazards on human health, the increasing importance and rapid growth of world food trade and the demand by consumers for a safe food supply, analysis of the risks associated with food has become more important than ever before.
Risk assessment is a quantitative evaluation of information on potential health hazards from exposure to various agents.
It involves four interrelated steps:
The entire risk assessment process requires the use of sound and scientifically derived information and the application of established scientific procedures carried out in a transparent manner.
Unfortunately, sound scientific data are not always available for the qualitative and quantitative evaluations necessary for an absolutely sure final decision; consequently a degree of uncertainty must be factored into the decision.
The importance of risk assessment lies not only in its capacity for estimating human risk, but also in its function as a framework for organizing data as well as for allocating responsibility for analysis.
The risk assessment process can include a variety of models for reaching conclusions; for example, the concept of acceptable daily intake (ADI) may be considered a component of risk assessment.

Legal requirements, International Standards and Private Voluntary Standards
The foundations of the diferent Food Safety Management System is HACCP.
The HACCP system is the system that is required for any food business or organisation in most countries by legislation.
The joint FAO / WHO Codex Alimentarius Commission recommends the HACCP approach to enhance Food Safety.

The HACCP system was originally developed for use in aerospace manufacturing under the name “Failure Mode Effect Analysis”. It was first adapted to food processing by the Pillsbury Company in 1959 in a project for the NASA space program. At that time, HACCP was used to guarantee that food used in the U.S. space program would be 100% free of viral and bacterial pathogens.
By the late 1960s, Pillsbury was using a HACCP system to manufacture consumer goods.
In late 1989, the Food Safety and Inspection Service (FSIS) announced its intention to implement HACCP in meat and poultry inspection operations. FSIS embarked on a 3 year study to determine how best to implement HACCP.
Traditionally, the Hazard Analysis and Critical Control Point (HACCP) methodology has been considered to be a food safety management system.
It aims to prevent known hazards and to reduce the risks that they will occur at specific points in the food chain. The same principles are also increasingly being applied in other industries, such as the car industry, aviation and the chemical industry.
Hazards affecting quality are controlled to a certain extent through the validation of critical operations and processes in the manufacture of finished products in accordance with Good Manufacturing Practices (GMP).
However, GMP do not cover the safety of the personnel engaged in manufacture, while both aspects are covered by HACCP.
The following elements of the HACCP methodology are integral parts of the validation master file:
HACCP should not replace GMP; however, its application may be used as a first step towards GMP.
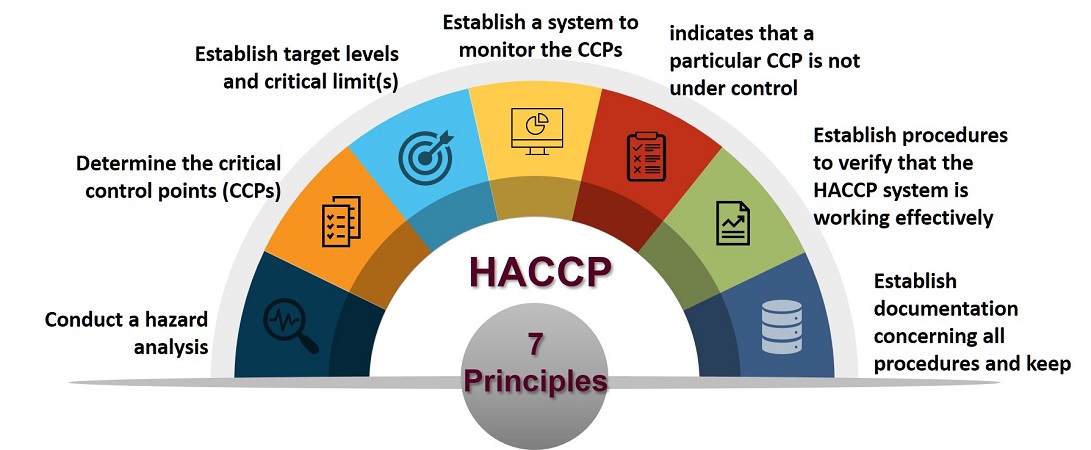
The HACCP system is based on seven principles.
In applying these principles, 12 stages are recommended and are discussed below.
Some stages are linked to specific principles while others serve as an introduction to the concept.
The following guidelines will be found useful in applying the HACCP system:
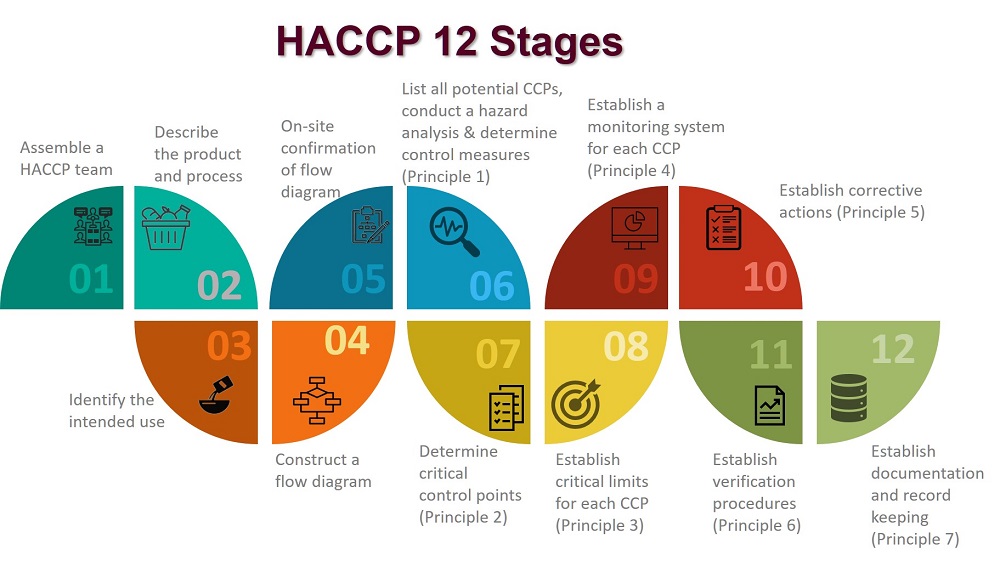
The application of HACCP principles consists of the 12 stages, as identified in the logic sequence for application of HACCP.
The pharmaceutical manufacturer should assure that product-specific knowledge and expertise are available for the development of an effective HACCP plan. This may be best accomplished by assembling a multidisciplinary team.
Team members should therefore represent all the relevant disciplines, such as research and development, production, quality control, quality assurance, microbiology, engineering and distribution or others as applicable. Team members should have specific knowledge and expertise regarding the product and process. Where such expertise is not available on site, expert advice should be obtained from other sources.
Team members should be able to:
The scope of the HACCP plan should be defined. The scope should describe the segment of the process involved and the classes of hazards to be addressed should be identified.
A full description of the product and the process should be drawn up, including relevant quality information such as the composition, physical/chemical properties, structure, pH, temperatures, method of cleaning, bactericidal/ bacteriostatic treatments (e.g. heat-treatment), drying, screening, mixing, blending, packaging, and the storage conditions. The method of distribution and transport should also be described, especially where products are thermolabile.
The intended use should be based on the expected uses of the product by the end-user or consumer. In specific cases, vulnerable population groups, e.g. geriatric patients, infants and immunocompromised patients, may have to be considered.
The flow diagram should be constructed by the HACCP team, and should cover all operations and decisions in a process.
When applying HACCP to a given operation, the steps preceding and following that operation should also be considered.
A block-type diagram may be sufficiently descriptive.
The HACCP team should confirm the processing operation against the flow diagram during all stages and hours of operation. Amendments to the flow diagram may be made where appropriate, and should be documented.
When hazard analysis is conducted, safety concerns must be distinguished from quality concerns.
The HACCP team should list all the hazards that may be reasonably expected to occur at each step from production, testing and distribution up to the point of use. It should then conduct a hazard analysis to identify for the HACCP plan which hazards are of such a nature that their elimination or reduction to acceptable levels is essential.
A thorough hazard analysis is required to ensure an effective control point. A two-stage hazard analysis is recommended. During the first stage, the team should review the materials, activities, equipment, storage, distribution and intended use of the product. A list of the potential hazards (biological, chemical and physical) which may be introduced, increased or controlled in each step should be drawn up.
In the hazard analysis, the following should be included wherever possible:
During the second stage, a hazard evaluation should be conducted, i.e. the severity of the potential hazards and the probability of their occurrence should be estimated.
The team should then decide which potential hazards should be addressed in the HACCP plan, and what control measures, if any, exist that can be applied for each hazard. More than one control measure may be required to control a specific hazard(s) and more than one hazard may be controlled by a specified control measure.
Potential hazards in relation to at least the following should be considered:
A CCP in the HACCP system can be more easily determined by the use of a decision-tree, which facilitates a logical approach. The way that a decision-tree is used will depend on the operation concerned, e.g. production, packing, reprocessing, storage, distribution. Training in the use of decision-trees should be given.
If a hazard has been identified at a step where control is necessary for safety, and no control measure exists at that step, or any other, the product or process should be modified at that step, or at an earlier or later stage, to include such a control measure.
Critical limits must be specified and verified, if possible, for each critical control point. More than one critical limit may sometimes be elaborated at a particular step. The criteria used often include measurements of temperature, time, moisture level, pH, and sensory parameters, such as visual appearance and texture.
Critical limits should be scientifically based.
Monitoring is the scheduled measurement or observation of a CCP relative to its critical limits. Monitoring should be recorded.
The monitoring procedures used must be able to detect loss of control at the CCP, and this information should ideally be available in time to make adjustments to ensure control of the process and prevent violations of the critical limits. Where possible, process adjustments should be made when the monitoring results indicate a trend towards loss of control at a CCP. These adjustments should be made before a deviation occurs.
Data derived from monitoring must be evaluated by a designated person with the knowledge and authority to carry out corrective actions when indicated.
If monitoring is not continuous, the amount or frequency of monitoring must be sufficient to guarantee that the CCP is under control.
Most monitoring procedures for CCPs will need to be done rapidly because they relate to on-line processes and there will not be time for lengthy analytical testing. For this reason, physical and chemical measurements are often preferred to microbiological tests because they can be done rapidly and can often indicate the microbiological control of the product.
The personnel conducting the monitoring of CCPs and control measures should be engaged in production (e.g. line supervisors, maintenance staff) and, where appropriate, staff from quality control. They should be trained in monitoring procedures.
Where continuous monitoring is possible, a reliable monitoring procedure and frequency should be identified. Statistically designed data collection or sampling systems should then be used.
All records and documents associated with monitoring CCPs must be signed and dated by the person(s) carrying out the monitoring and by a responsible reviewing official(s) of the company.
Specific corrective actions should be developed for each CCP in the HACCP system in order to deal with deviations when they occur. These actions should ensure that the CCP is brought under control. Corrective actions should include at least the following:
Specific corrective actions should be developed in advance for each CCP and included in the HACCP plan. As a minimum, this plan should specify what is to be done when a deviation occurs, who is responsible for implementing the corrective actions, and that a record will be kept and maintained of the actions taken. Individuals who have a thorough understanding of the process, product and HACCP plan should be assigned the responsibility for the oversight of corrective actions.
As appropriate, experts may be consulted to review the information available and to assist in determining the disposition of non-compliant product.
Actions taken must also include the proper disposition of the affected product.
Deviation and product disposition procedures must be documented in the HACCP records.
Procedures should be established for verification. Verification and auditing methods, procedures and tests, including random sampling and analysis, can be used to determine whether the HACCP system is working correctly. The frequency of verification should be sufficient to confirm the proper functioning of the HACCP system.
Examples of verification activities include:
Initial verification of the HACCP plan is necessary to determine whether it is scientifically and technically sound, that all hazards have been identified, and that, if the HACCP plan is properly implemented, these hazards will be effectively controlled.
Information reviewed to verify the HACCP plan should include:
For example, verification of the moist heat sterilization process for sterile injectables should include the scientific justification of the heating times, pressure and temperatures needed to obtain an appropriate destruction of pathogenic microorganisms (i.e. enteric pathogens) and studies to confirm that the sterilization conditions ensure that the whole load is kept at the required temperature for the time required.
Subsequent verifications should be performed and documented by a HACCP team or an independent expert, as needed. For example, verifications may be conducted when there is an unexplained system failure, a significant change in product, process or packaging occurs, or new hazards are recognized.
In addition, a periodic comprehensive evaluation of the HACCP system by an unbiased, independent third party is useful. This should include a technical evaluation of the hazard analysis and each element of the HACCP plan as well as an on-site review of all flow diagrams and appropriate records of the operation of the plan. Such a comprehensive verification is independent of other verification procedures and must be performed in order to ensure that the HACCP plan is resulting in the control of the hazards. If the results of the comprehensive verification identify deficiencies, the HACCP team should modify the HACCP plan as necessary.
Individuals doing verification should have appropriate technical expertise to perform this function.
Where possible, verification should include actions to confirm the efficacy of all elements of the HACCP plan.
Efficient and accurate documentation and record keeping are essential to the application of a HACCP system and should be appropriate to the nature and size of the operation.
Examples of activities for which documentation is required include:
Examples of activities for which records are required include:
For more details, please visit our Recall Section.
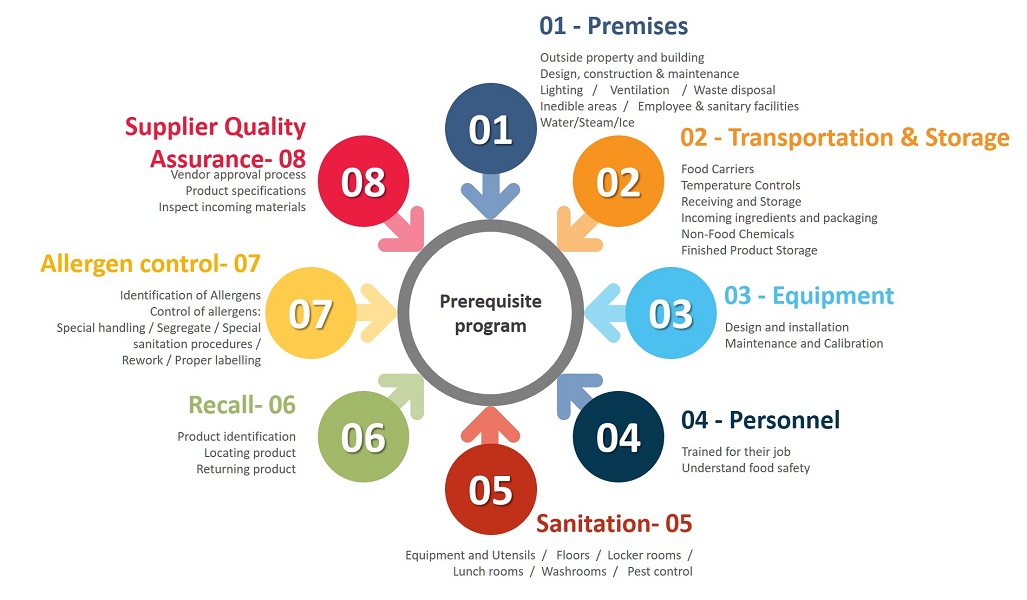
For the importance of Sanitation, Recall and Allergen Control, we procced to detail these 3 Prerequisites.
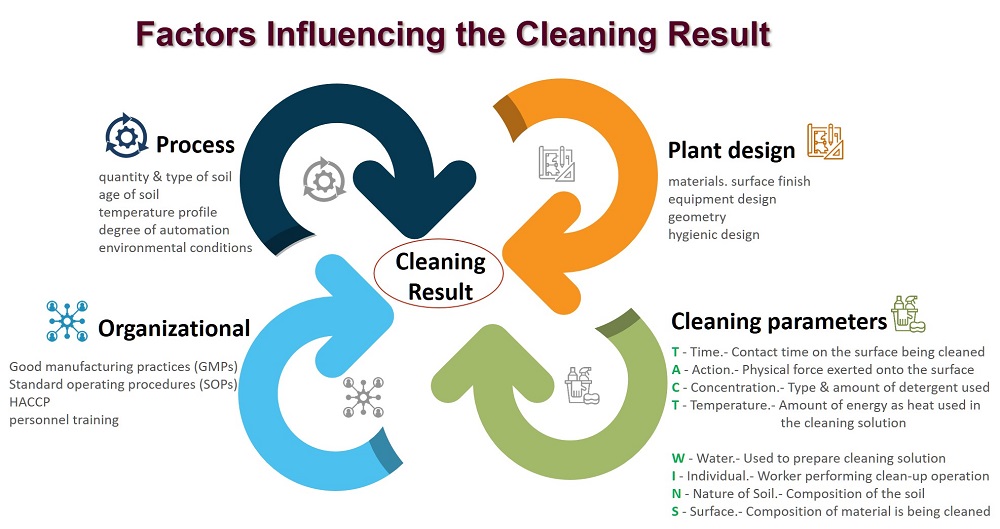
What is "Sanitation"? - The process of creating conditions that promote the safe production of food.
The broad term 'Sanitation' can be divided into two components:

Multiple Step Process
Each of these steps must be done – in this order – for the process to be effective.
These two steps are two completely separate operations; you must clean a surface before sanitizing can be effective. Each step is important, for different reasons, in achieving food safety.
A clean surface is needed so that the bacteria or viruses will be killed by the action of the sanitizer. If a sanitizer is applied to a surface that has not been ‘cleaned’ – its action will not be effective against the microbes.
While microbes are killed in the sanitizing step, food allergens are controlled at the cleaning/washing step.
Each type has a specific function – choose an appropriate product for your needs
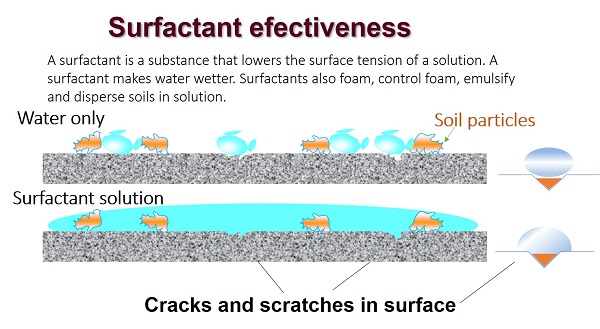
You must use the proper type of cleaner in correct proportions for each cleaning task.
There are some disadvantages with some types of cleaners; they may react with some types of surfaces. For example, highly alkaline detergents shouldn’t be used on aluminum pans or cooler walls because they will pit the surface.
Following these steps will result in a clean surface!
The temperature of water is specific to the type of sanitizer being used. For example, water that is too hot can cause chlorine to evaporate from the solution.
Allow the equipment and utensils to remain in the sanitizing solution for enough time. The proper time will vary depending on the sanitizer that is being used.
THE KEY IS THAT YOU MUST FOLLOW THE LABEL USE INSTRUCTIONS for the sanitizer you use.
DO NOT MIX CHEMICALS!
Is determined by many factors like:
Sanitation is everyone’s responsibility! And training is critical; employees can’t do what they don’t know!
Written Procedures
When problems are found, solve the problem and retrain the employees with the proper procedures. Encourage employee feedback to improve procedures. Communication and follow-up are key to effective monitoring
Food allergens are truly the newest food safety hazard.
It is a protein in some foods that cause allergic reactions in some people.
The USFDA clasiffied eight food groups that are the cause of 90% of food allergic reactions. Those 8 food groups are milk, eggs, peanuts, tree nuts, wheat, soy, fish, and shellfish
México and the European Union they include more products in their regulations.
Foods must be labeled accurately. It is critical that food labels contain a complete list of ingredients that declare all allergens.
Effective cleaning procedures eliminate residues that cause food allergies, avoid cross contamination.

For a more detailed presentation please visit our Recall Section.
Espiga Exports has the experience in Food Safety Management System, Recalls, Traceability, Audits and relevant food regulations in the European Union, the Unitede States and Mexico and could provide a wide range of consulting servicies tailored to your company.
Espiga Exports has the experience in Food Safety Management System, Recalls, Traceability, Audits and relevant food regulations in the European Union, the Unitede States and Mexico and could provide a wide range of consulting servicies tailored to your company.
We are ready to exceed your expectations and help you achieve your goals.